Abstract
Introduction
IL-10-producing regulatory B cells (Bregs) are important modulators of immune regulation, and in humans this population has been shown to be enriched within the CD19+CD24hiCD38hi B cell population in peripheral blood. The production of IL-10 by Bregs can be induced through activation of B cells through toll like receptor (TLR) and B cell receptor (BCR) ligation, or indirectly through T cell-mediated activation via CD40:CD40L interactions. Loss of Breg-mediated suppression, either through numerical deficiency or loss of function, has been implicated in the pathogenesis of several autoimmune and inflammation-mediated diseases, including chronic Immune Thrombocytopenia (ITP; Li et al, 2012, Blood, 120:3318-3325). The purpose of this study was to extend this observation by interrogating the effect of systemic immunosuppressive treatment, in particular with glucocorticoids (GCs), on Bregs from the peripheral blood of patients with ITP.
Methods
Following informed written consent, peripheral blood was drawn from patients with ITP registered at University Hospitals Bristol, UK, and healthy volunteers. Peripheral blood mononuclear cells were isolated by density gradient centrifugation, and multiparameter flow cytometry performed to quantify the frequency of CD19+CD24hiCD38hi B cells. For functional studies, CD19+ B cells and CD4+ T cells were isolated from the peripheral blood of healthy donors using fluorescence activated cell sorting (FACS) and subsequently co-cultured in a 1:1 ratio. Cells were activated with either anti-CD3 (indirect B cell activation via T cells) or a combination of CpG and IgM (direct B cell activation), in the presence or absence of the synthetic GC Dexamethasone (Dex). After 72 hours, intracellular IL-10 expression in B cells was quantified by flow cytometry.
Results
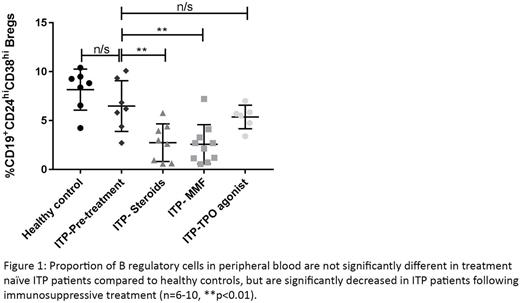
No significant difference in the proportion of CD19+CD24hiCD38hi Bregs was seen between newly diagnosed ITP patients (n=7) before treatment had started, compared with healthy donors (n=7), (6.5% vs 8.2%, p=0.3176; Figure 1). However, patients receiving GC treatment (n=8) had a significant reduction in the proportion of Bregs when compared with both healthy donors (2.5% vs 8.2%, p=0.0012), and untreated ITP patients (2.5% vs 6.5%, p=0.0093). Similarly, ITP patients receiving another commonly used immunosuppressive drug, mycophenolate mofetil (MMF, n=10), had a significant reduction in the proportion of Bregs compared with both healthy donors (1.7% vs 8.2%, p=0.0004), and untreated ITP patients (1.7% vs 6.5%, p=0.0042). Patients treated with a thrombopoietin (TPO) mimetic (n=6) (not immunosuppressive) had no reduction in the proportion of Bregs compared with untreated ITP patients (5.37% vs 6.49% p=0.4452). Furthermore, in cultures activated by CpG and IgM, Dex did not significantly affect the number of IL-10-producing B cells (5.4% vs 5.4%, p=0.9232, n=10). However, in anti-CD3 activated cultures, Dex significantly reduced the proportion of IL-10 producing B cells (2.0% vs 0.8%, p=0.0094, n=8).
Conclusions
These observations suggest that a deficiency of Bregs in the peripheral blood is not a result of disease activity in ITP patients, but rather the consequence of immunosuppressive drug treatment. Consistent with this, the effect of Dex on B cell IL-10 production is likely to be an indirect phenomenon through the suppression of T cell-mediated activation of Breg function. Overall, these observations show that immunosuppressive treatments such as GCs can deplete Breg numbers in the peripheral blood and, through a T cell mediated mechanism, reduce their ability to produce the immunoregulatory cytokine IL-10.
Bradbury: BMS Pfizer: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Other: Travel support; Bayer: Other: Conference attendence; Amgen: Other: Conference attendence. Lee: University of Bristol: Patents & Royalties: International Patent Application No: PCT/GB2014/053804 / US Patent application No. 61/919,404 (A biomarker and companion therapeutic for steroid refractory diseases); EMD Serono: Consultancy; Nanomerics: Consultancy; Roche: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal